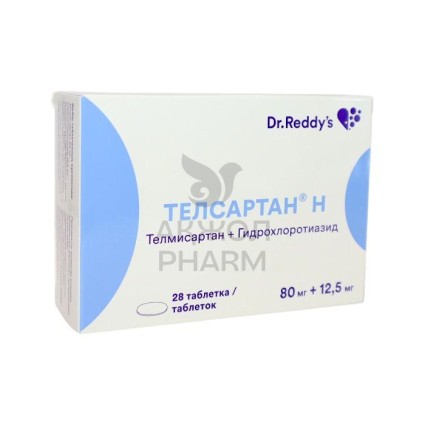
Indian pharmaceutical company Dr. Reddy's Laboratories announced a recall of a batch of Telsartan N, the Center for Pharmaceutical Safety reported.
quality control and stability testing conducted by the manufacturer found that the batch C2501976 of Telsartan N 80 mg/12.5 mg has a deviation in the active ingredient content, hydrochlorothiazide, from standards. This is deemed a non-compliance with quality requirements for the drug.
Batch C2501977, put into circulation in Uzbekistan, was not found to have such non-compliance with the active ingredient content. However, since this batch was manufactured using an active ingredient from the same production lot as the batch with the identified non-compliance, this batch is being preventively withdrawn from the market to ensure safe circulation of the drug and prevent potential risks.
Individuals who have purchased Telsartan H 80 mg/12.5 mg from the above-mentioned series are advised not to use it and to consult a doctor to select an alternative medication, if necessary.